A team of researchers at Helmholtz Zentrum München, LMU University Eye Hospital Munich, and the Technical University of Munich (TUM; all in Munich, Germany) has developed a deep learning method that makes automated screenings for eye diseases such as diabetic retinopathy more efficient. The method has the ability to reduce the amount of expensive annotated image data that is required for the training of the algorithm, making it ideal for clinical use. For diabetic retinopathy screening, the researchers developed an algorithm that needs 75% less annotated data and achieves the same diagnostic quality of human experts.
In recent years, clinics have taken first steps towards artificial intelligence (AI) and deep learning to automate medical screenings. However, training a deep learning algorithm for accurate screening and diagnosis prediction requires large sets of annotated data and clinics often struggle with expensive expert labeling. Researchers were therefore looking for ways to reduce the need for costly annotated data while still maintaining the high performance of the algorithm.
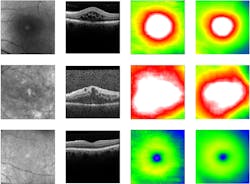
Diabetic retinopathy, a diabetes-related eye disease that damages the retina and can ultimately lead to blindness, requires measurement of the retinal thickness to diagnose the disease. To do so, most clinics take photographs of the fundus (the surface of the back of the eye). In order to automate the screening of these images, clinics started to apply deep learning algorithms. These algorithms require large sets of fundus images with expensive annotations in order to be trained to screen correctly. LMU University Eye Hospital Munich owns a population-size dataset containing over 120,000 unannotated fundus and co-registered optical coherence tomography (OCT) images. OCT allows for precise information about the retinal thickness, but is not commonly available in every eye care center. LMU provided their data to researchers from Helmholtz Zentrum München who are pioneering in the field of AI in health.
“Our goal was to use this uniquely large set of fundus and OCT images to develop a method which will reduce the need of expensive annotated data for algorithm training,” says Olle Holmberg of Helmholtz Zentrum München and the TUM School of Life Sciences, the first author of the paper that details the work. The research team developed a novel method called “cross modal self-supervised retinal thickness prediction” and applied it to pre-train a deep learning algorithm with the LMU dataset. Cross modal self-supervised learning allowed the algorithm to teach itself to recognize unannotated fundus images with different OCT-derived retinal thickness profiles, predicting the thickness information directly from the fundus. By accurately predicting retinal thickness, a key diagnostic feature for diabetic retinopathy, the algorithm was then able to learn how to predict screening outcomes.This novel method shrunk the need for expensive annotated data to train the deep learning algorithm significantly. When applied in automated screenings for diabetes retinopathy, it achieved the same diagnostic performance compared to previous algorithms that had required much more training data and compared to human experts.
“We reduced the need for annotated data by 75 percent,” states Prof. Fabian Theis, who led the study as Director of the Institute of Computational Biology at Helmholtz Zentrum München and Scientific Director of Helmholtz AI, the AI platform of the Helmholtz Association. “Sparse annotated data is a grand challenge in medicine. It is one of our goals to develop methods that work with less data and that can then potentially be applied in many settings. Our use case in diabetic retinopathy is ready for immediate use in clinics and is a perfect example of how AI can improve the daily business of clinics and thus everybody’s health.”
“Automated detection and diagnosis of sight-impairing diabetic retinopathy with widely available fundus photography is a big improvement for screenings. Patient referrals to partly overcrowded specialized eye care centers could thus be reduced as well,” says Dr. med. Karsten Kortuem, LMU University Eye Hospital Munich, who was responsible for the clinical side of this study. Moreover, an additional reduction in size, meaning number of parameters, was achieved in the algorithm itself. The novel method enables up to 200 times smaller algorithms. This could be a crucial benefit to deploying them on mobile and embedded devices which is also important in clinical settings.
Beyond diabetic retinopathy, the novel method allows for further clinical applications where much unannotated data is available but expert annotations are scarce, such as age-related macular degeneration (AMD).
The self-supervised pre-trained algorithm from this study is available on https://github.com/theislab/DeepRT.
Full details of the work appear in the journal Nature Machine Intelligence.