Chemical and morphological analysis of live cells and subcellular components are cornerstones of biological sciences. Combining these techniques to produce two- and three-dimensional chemical images of cells and organelles has enabled a more in-depth understanding of cellular function, leading to many of the breakthroughs of modern biology and medicine. Over the past 40 years, lasers have played an invaluable role in developing high-resolution chemical imaging techniques, especially those based on Raman and fluorescence spectroscopy. This article presents a brief historical review of laser microscopy along with a simultaneous discussion of how the laser technology of the time influenced the dominance of specific techniques, with a focus on ultrafast laser systems.
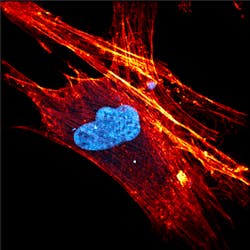
Nonlinear processes such as multiphoton fluorescence and coherent Raman microscopies offer a wide variety of advantages over traditional Raman and fluorescence cell imaging. These techniques are especially promising due to their sensitivity, resolution, and ability to operate with or without labeling. Even though these techniques were first experimentally demonstrated more than 30 years ago, the need for ultrafast laser sources has limited their commercial viability. Luckily, the recent boom in compact mode-locked fiber and disk lasers is finally allowing these technologies to move from physics and engineering labs to the biology and biochemistry ones. For example, Figure 1 shows a high-resolution multiphoton image of a stem cell measured using the FemtoFiber ultra 920 by TOPTICA Photonics (Munich, Germany).
History of laser microscopy
Two-photon fluorescence spectroscopy, coherent anti-Stokes Raman spectroscopy (CARS), and stimulated Raman spectroscopy (SRS) are specific subsets of multiphoton fluorescence and coherent Raman microscopies, which themselves are subsets of fluorescence and Raman spectroscopies. Therefore, it is essential to take a look at the development of all of them simultaneously to understand the evolution of laser microscopy as a whole. The earliest use of laser microscopy in biological sciences was reported in 1982 by Michael Duncan and others at the Naval Research Laboratory (Washington, DC) to produce CARS images of onion cells.1 At that time, it was necessary to use two dye lasers pumped by a mode-locked argon-ion laser to provide ultrafast tunable pump (565–620 nm) and Stokes (620–700 nm) excitation sources.
Ironically, it wasn’t until two years later that the first scanning confocal fluorescence microscope capable of imaging living cells was constructed by Ingemar Cox at Oxford University (Oxford, England).2 While lacking the chemical specificity of Raman microscopy, using fluorescence spectroscopy greatly simplified the laser requirements, enabling Cox to image hamster kidney cells stained with fluorescein isothiocyanate (FITC) using a 10 mW HeCd laser at 441.6 nm. The simpler laser requirements resulted in confocal fluorescence microscopy becoming the dominant cell-imaging methodology for a long time, despite the sample preparation and potential photochemical degradation.
Still, research into nonlinear microscopy techniques continued, and in 1985, Isaac Freund and Moshe Deutsch at Bar-Ilan University (Ramat-Gan, Israel) published the first second-harmonic generation (SHG) microscopy images of biological tissues.3 Using a Q-switched Nd:YAG laser, they produced images of collagen fibrils in rattail tendons, showing the ability to detect fibril orientation and thickness. The resolution was relatively coarse (about 50 µm), but their work showed great promise for developing label-free chemical images for cell biology.
Five years later at Cornell University (Ithaca, NY), Winfried Denk, James Strickler, and Watt Webb took nonlinear microscopy to the next level with their pivotal work on two-photon fluorescence microscopy images of live cells, using a mode-locked dye laser with a pulse width of approximately 100 fs.4 While the advantages of switching from using a near-infrared excitation source for the imaging of living cells was evident from the beginning, the laser sources of the time were still too cumbersome for mass adoption of the technique. Even as self-mode-locked Ti:sapphire lasers replaced dye lasers throughout the 1990s and early 2000s, they were still too expensive, large, and problematic for widespread commercialization. Luckily, over the past decade, there has been a renaissance in ultrafast-laser technology with the commercialization of mode-locked fiber and disk lasers, significantly reducing the cost and complexity of ultrafast laser sources. For example, the laser head for the TOPTICA Photonics FemtoFiber ultra 920 (see Fig. 2) is only 77 × 165 × 300 mm in size and weighs 4 kg, in stark comparison to a typical mode-locked Ti:sapphire laser, which can be more than 40X larger.It is also interesting to note that in the same year that two-photon microscopy was first demonstrated, Gerwin Pupples and a joint team of researchers from the University of Twente (Enschede, Netherlands) and the Max Planck Institute (Göttingen, Germany) developed the first confocal Raman microscope capable of imaging a single living cell.5 Confocal Raman provided excellent spatial resolution (better than 1 µm3) and superior chemical specificity. Unfortunately, confocal Raman suffers from low sensitivity because it depends on spontaneous Raman scattering. Despite its initial promise, it would take another 15 years until the laser and detector technologies developed initially for telecom would enable confocal Raman to compete with confocal fluorescence.
Current state of nonlinear microscopy lasers
Unlike Ti:sapphire, which is highly tunable between roughly 700 to 900 nm with a peak efficiency near 780 nm, most mode-locked fiber and disk lasers operate in the 900–1100 nm range. This spectral range corresponds nicely with the two-photon absorption bands of most fluorescent proteins of interest to biologists. Near-infrared excitation wavelengths also have advantages of high depth penetration and low phototoxicity, making them ideal for imaging living tissue. Fabian Voigt and collaborators from the University of Zurich (Zurich, Switzerland) were among the first to demonstrate the advantage of longer wavelength excitation. They developed a 1027 nm passively mode-locked diode-pumped semiconductor disk laser that showed comparable multiphoton imaging performance to a Ti:sapphire laser in early 2017.6 One of the perceived drawbacks of mode-locked fiber and disk lasers is that they operate at a single output wavelength. Today, however, turnkey off-the-shelf solutions are like the picoEMERALD S from Applied Physics & Electronics (Berlin, Germany) are available with a built-in optical parametric oscillator (OPO).
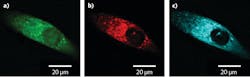
A recent example was published earlier this year by Liam Wilson, William Tipping, and others at the University of Strathclyde (Glasgow, Scotland) when they used SRS to map the cytoplasmic distribution of an alkyne-based reporter molecule for intercellular pH sensing.7 Figure 3 shows high-resolution SRS images of a prostate cancer cell treated with the reporter molecule. The green image represents the intensity distribution of the CH3 stretch (2933 cm-1), which is indicative of protein concentration, red shows the CH2 stretch (2851 cm-1) corresponding to lipid concentration, and cyan represents the alkyne band (2221 cm-1) of the pH reporter molecule demonstrating the ability to probe for intracellular pH. A dual-output picoEMERALD, producing a Stokes beam at 1031.4 nm and a tunable pump beam from 700 to 900 nm with a 2 ps pulse width, was used to record all three SRS images.Another up-and-coming methodology for the tuning of ultrafast lasers is soliton self-frequency shifting (SSFS), which enables an all-fiber tunable laser. While the theory behind SSFS in fiber optics has been established since the mid-1980s,8 the commercial implementation of SSFS is only just beginning. Last summer, as part of a joint effort between TOPTICA Projects (Graefelfing, Germany) and OFS Laboratories (Somerset, NJ), an all-fiber tunable mode-locked laser was developed with up to 18 nJ of pulse energy and a 120 fs pulse width, tunable from 1620 to 1990 nm.9 In the same article, Thomas Hellerer from the University of Applied Sciences Munich (Munich, Germany) used this laser (in conjunction with external SHG and OPO) to generate extremely high-resolution CARS images of mouse-fat tissue and two-photon images of a single mouse fibroblast cell stained with various fluorophores.
Looking to the future
According to Mordor Intelligence’s recent market analysis, as of 2019 the total value of the ultrafast laser market was an astounding $1.44 billion and was projected to have a compound annual growth rate (CAGR) of 12.7% over the next five years.10 A different market analysis firm, Fact.MR, projected a more-aggressive 14% CAGR, even after the outbreak of COVID-19—the firm stated that in 2019, bioimaging made up 30% of the overall market for ultrafast lasers.11 However, it should be noted that fact.MR did project a slightly smaller overall market cap.
With all of the money and resources expected to pour into ultrafast laser development over the next several years, it is clear that the trends toward compact, user-friendly, low-cost systems will only accelerate, resulting in an ever-increasing adoption of nonlinear laser microscopy in the biological and biomedical communities, particularly with regard to coherent Raman and multiphoton fluorescence microscopy.
REFERENCES
1. M. D. Duncan, J. Reintjes, and T. J. Manuccia, Opt. Lett., 7, 8, 350–352 (1982).
2. I. J. Cox, J. Microsc., 133, 2, 149–154 (1984).
3. I. Freund and M. Deutsch, Opt. Lett., 11, 2, 94–96 (1986).
4. W. Denk, J. H. Strickler, and W. W. Webb, Science, 248, 4951, 73–76 (1990).
5. G. J. Puppels et al., Nature, 347, 6290, 301–303 (1990).
6. F. F. Voigt et al., Biomed. Opt. Express, 8, 7, 3213–3231 (2017).
7. L. T. Wilson et al., Analyst, 145, 15, 5289–5298 (2020).
8. J. P. Gordon, Opt. Lett., 11, 10, 662–664 (1986).
9. A. Zach et al., Opt. Lett., 44, 21, 5218–5221 (2019).
10. See https://bit.ly/MordorInt.
11. See https://bit.ly/FactMr.